External Anatomy
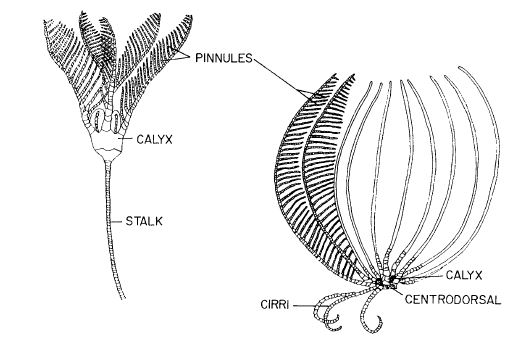
Figure 7 - Body plans of Crinoids (Feather Stars), from Ruppert et al (2004)
Body Form and Symmetry
One of the defining characteristics of echinoderms is their radial or pentamerous symmetry which evolved within the phyla, and describes the echinoderm body plan as being able to be divided into five similar parts around a central axis (Rupert et al 2004). This pentaramous symmetry can be seen in the pictures below in regards to the placement of the arms (Figure 6). Echinoderm larvae vary from this symmetry by having bilateral symmetry, and in the case of crinoid larvae, have a barrel shape encircled by four or five bands of cilia (Ruppert et al 2004).
The typical crinoid body consists of a stalk, for attachment, that supports a pentamerous crown, which includes the central disc/calyx cup and arms (Ruppert et al 2004 - Figure 7). In the case of comatulid crinoids (Feather stars), such as C. glebosus, the stalk is lost during post larval development, with the cirri closest to the central disc remaining to form a circle around the base of the crown (Ruppert et al 2004).
An epidermis covers an ossicle embedded connective tissue layer across all crinoid body parts, with the ossicles acting as a skeleton to help stiffen the body (Ruppert et al 2004). Inthe arms, cirri and pinnules the relationship between the embedded ossicles and the musculature system allow for a varying range of movements and states that allow for either rigidity or fluidity (Ruppert et al 2004).
The crinoid cup consists of two parts the aboral calyx and the tegmen (Ruppert et al 2004). The aboral calyx is a heavily calcified cup that holds the viscera, while the tegmen is the membranous oral wall of the disc that is weakly calcified and covers the calyx like a lid top on the oral or dorsal side of the crinoid (Ruppert et al 2004).
The mouth sits on the border of the tegmen with five ambulacral grooves, a furrow containing tube feet,radiating out from the mouth and along the arms (Ruppert et al 2004).The anus also opens out onto the oral surface but in an interambulacrum, an area in-between the ambulacra. It sits offset on the top of a prominence called the anal cone, which allows for elevation above feeding currents to ensure prevention of fouling of food and feces moving into the mouth (Ruppert et al 2004).

Figure 6- Pentaramous symmetry of C. glebosus, collected off Amity Point on North Stradbroke Island. Photo taken by Sophie Horsfall
Cirri
Comatulid crinoids have elongated, highly ossified, jointed appendages called cirri on their aboral or downward facing surface of their body that allows for attachment to substrates (Holland and Grimmer 1981). This positioning of the cirri on the aboral surface of the crown allows for the oral surface to be directed upwards (Ruppert et al 2004). Cirri can move slowly by either bending downwards to form an arc or unbending upwards to form a straight surface this allows for grasping of substrates to anchor or slow movement (Clark 1915, Holland and Grimmer 1981). Depending on the type of substrate the length and shape of the cirri varies. Species that cling to rocks, coral, seaweed and other objects have been found to have short,stout, curved cirri that are adapted to grasping objects, and are mainly in comantulid crinoids (Ruppert et al 2004, Alender et al 1966). Long, slender cirri have been found on species that live in soft, mud bottom environments and help prevent the animal from sinking into the substrate (Alender et al 1966, Ruppert et al 2004). While long, robust cirri are present in crinoids that live on hard bottom substrates without rocks and coral (Alender et al 1966 - Figure 8).
The muscular, nervous, internal transport and skeletal structures of cirri include rows of calcareous skeletal ossicles, oral and aboral ligaments, cirral nerves, oral and aboral coeloms, heamal canals, soft dermis and thin epidermis, and neuro-secretory neurons; see Holland and Grimmer (1981) for further information.

Figure 8- The cirri of C. glebosus, crinoid appendages used for mobility and attachment. Photo taken by Sophie Horsfall.
Substrate Preference Test
Using the knowledge of cirri shape and size and their relationship to substrate types, three C. glebosus individuals were tested for substrate preference in an aquarium setting. C. glebosus has relatively short and robust cirri (Figure) implying the species would be tailored towards rocky, coral habitats with hard arborescent growths (read above).
For each test a C. glebosus individual was placed within a rectangular aquarium half filled with a rocky, coral substrate with the other half left void of materials, for 15 minutes. Each individual was tested twice for their substrate preference, with the rocks either at the front or the back of the aquarium to eliminate any possible effects that could be due to differences in light intensity or human movement. The tank was full to its capacity of 37.5 litres which allowed the crinoid to be fully submersed into the water, even on top of the rocks, to ensure that water level was not a confounding factor. Although the tank was slowly filtered there was no main water current or flow that would affect the placement preference of the individual within the tank. Each individual was placed into the middle of the tank with half of their arms on the rocky substrate and half of their arms on the aquaria glass to ensure knowledge of substrate availabilities within the tank.
In all tests the C. glebosus individuals all choose the rocky substrate over the empty side of the tank. This correlates with previous studies on crinoids and the cirri shape and size with short, robust cirri designed for rocky substrates (Figure 8), but more testing should be done on C. glebosus in relation to the numerous substrate types available within Moreton Bay and their exact substrate preference. As there has been little to no observational or experimental studies on C. glebosus since its discovery further studies are suggested in all areas including behaviour, life history, abundance, chemical defense, anatomy and physiology to ensure its protect and longevity into the future.
Arms, Pinnules and Ambulacral Grooves
Five crinoid arms arise from the perimeter of the pentaramous crown and bear pinnately arranged, jointed appendages called pinnules to produce a feather like look, which gives rise to its common name, Feather Star (Ruppert et al 2004 - Figure 9). The five arm bases of most crinoids diverge to form ten arms, with further branching occurring in some species to produce additional arms (Ruppert et al 2004). The length of the arms range in length depending on the species but are usually between 10 and 25cm in length (Ruppert et al 2004). As previously mentioned, members of C. glebosus have arms of similar size. Each arm then contains a bilateral series of pinnately arranged, jointed appendages called pinnules (Ruppert et al 2004).
The five ambulacral grooves running along the tegmen (oral surface) diverge and run along the oral/dorsal side of the arms and pinnules (Ruppert et al 2004). These grooves house trios of tube feet that are joined at their bases, underneath a movable flat called a lappet that can expose or cover the groove (Ruppert et al 2004). Crinoid arms, especially in comatulids, function as not only appendages for suspension feeding but also play a role in locomotion (see Life History and Behaviour).
The musculature and skeletal components within crinoids arms include calcareous, calcium carbonate, plates called brachials that reside in the endoskeleton, ligaments that stiffen or soften to either lock these plates into a fixed pattern or allow plates to move relative to one another, highly developed muscular articulations and ligamentary articulations called syzygies that limit active mobility and have been found to be connected to the autonomy of arms in comatulids (Simms 1988, Emson and Wilkie 1980, Meyer 1971); see Holland and Grimmer (1981) and Oki and Okamoto (1994) for further information.

Figure 9 - The arms and pinnules of C. glebosus. Photo taken by Sophie Horsfall
Tube Feet
The tube feet found within the ambulacral grooves are external fluid-filled muscular tubes that make up part of the water vascular system and play a role in locomotion, food handling and respiration (Ruppert et al 2004).
Family members of Comasteridae, such as C. glebosus, have been found to have longer and more widely spaced tube feet compared to non-comasterids (Meyer 1979). Compared to non-comasterids four to six sets of tube feet perpinnular ossicle, comasterids only have 3 sets of tube feet per pinnular ossicle (Meyer 1979). These differences in tube feet morphology have been discovered to have a consistent relationship with crinoid living and feeding positions (Meyer 1979). See Life History and Behaviour – Feeding for more information.
|